Spotlight On: Fibroids

This month we cast a spotlight on articles, SurgeryU videos, and Journal of Minimally Invasive Gynecology (JMIG) article recommendations from the AAGL Fibroids Special Interest Group (SIG) led by Chair Jin Hee (Jeannie) Kim, MD, FACOG.
Access to SurgeryU and JMIG are two of the many benefits included in AAGL membership. The SurgeryU library features high-definition surgical videos by experts from around the world. JMIG presents cutting-edge, peer-reviewed research, clinical opinions, and case report articles by the brightest minds in gynecologic surgery.
SurgeryU video recommendations by our SIGs are available for public access for a limited time. The links to JMIG article recommendations are accessible by AAGL members only. For full access to SurgeryU, JMIG, CME programming, and member-only discounts on meetings, join AAGL today!
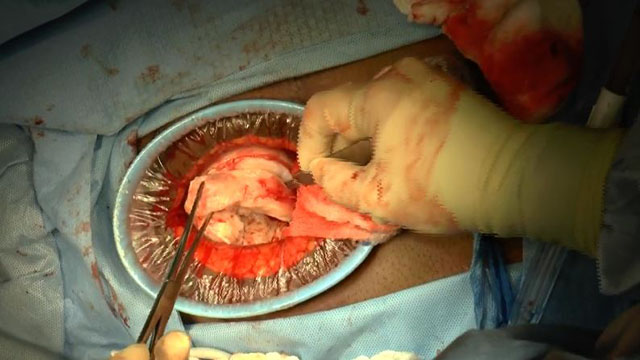
SurgeryU Video Recommendation #1:
Laparoscopic Cervical Myomectomy Techniques by Drs. Renee Sullender, Shira Varon, and Charlotte Pickett
Cervical fibroids can pose a significant challenge, particularly when there is a goal to preserve the uterus. Excessive bleeding is one of the primary concerns during myomectomy surgery. In this video, we can observe various approaches to minimize intraoperative bleeding, employing the appropriate techniques captured in high-quality images.
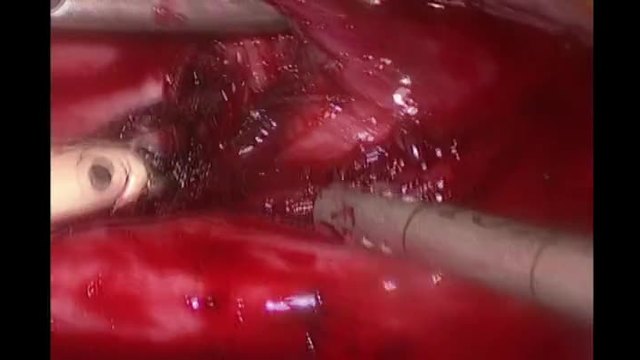
SurgeryU Video Recommendation #2:
Minilaparotomy: Abdominal Myomectomy by Drs. Sierra J. Seaman, Patricia J. Mattingly, and Arnold P. Advincula
Today, it is more important than ever to understand the needs of our patients and propose the best therapy individually, based on their expectations. Recognizing the limitations of each technique is the responsibility of a versatile surgeon who can offer patients minimally invasive outcomes through the utilization of various techniques and approaches, including vaginal or even mini-laparotomy procedures.
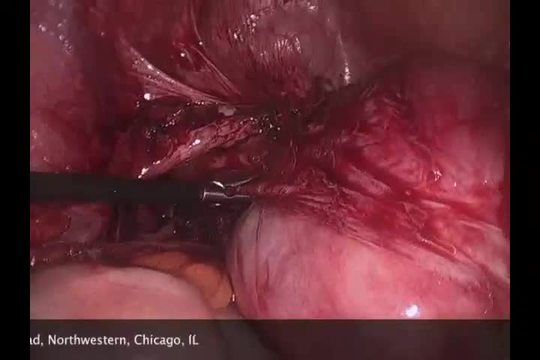
SurgeryU Video Recommendation #3:
Tips, Tricks, and Risks of Laparoscopic Myomectomy: Presented with a Case of a Broad Ligament Fibroid by Drs. Chris J. Kliethermes and Mary B. Holloran-Schwartz
The management of broad ligament fibroids can present challenges. Given their proximity to noble structures within the lateral compartment, such as the ureter and uterine vessels, complications can arise, demanding preparedness for their management. It is crucial to remember and not underestimate the inherent risks associated with surgical procedures.
SurgeryU Video Recommendation #4:
Cervical Myomectomy by Drs. Stacy M. Syrcle, Jennifer L. Eaton, and Magdy P. Milad
It is important to bear in mind that leiomyomas can also occur within the residual uterine cervix stump, particularly in cases where a woman presents with a solid pelvic mass after undergoing a subtotal hysterectomy. Accurate recognition of the anatomy and precise identification of planes are essential for the successful execution of myomectomy surgery in these cases.
About the Author:
Daniel S. Dias, MD, PhD

JMIG is a constant source of new evidence-based information to guide our practices. I am happy to share my 2 favorite fibroid related articles published in JMIG in 2023. Although very different articles, they both are easy to digest and start incorporating into your clinical practice.
JMIG Article Recommendation #1:
This article uses the large NSQIP database to look at transfusion risk for patients undergoing a myomectomy either open or minimally invasively. Data from over 26,000 women was analyzed to compare risk of transfusion at various pre-operative hematocrit levels. Figures 1 and 2 allow you to quickly reference your patient’s pre-op hematocrit and fibroid burden to give an estimate of her risk of transfusion.
Journal of Minimally Invasive Gynecology, Volume 30, Issue 2, p115-121, February 2023, DOI:https://doi.org/10.1016/j.jmig.2022.10.010.
JMIG Article Recommendation #2:
This is a rare qualitative study of fibroid patients in which semi-structured interviews were conducted with patients undergoing treatment for fibroids. This study emphasizes that patients are spending a significant amount of time doing their own research on fibroid treatment options outside of doctor’s appointments and are looking for social support on social media. Patients in the study also expressed a strong preference for knowing all their treatment options before deciding about their care. This study highlights that we need to address the full range of fibroid treatment options with patients (even if they are not good candidates for a particular procedure), because patients are coming into appointments understanding what is available from their online communities.
Journal of Minimally Invasive Gynecology, Volume 30, Issue 4, p284-289, April 2023, DOI:https://doi.org/10.1016/j.jmig.2022.12.008
About the Author:
Kristen Pepin, MD, MPH

Most Difficult Case: Coexistent Fibroids, Bilateral Endometriomas and Adenomyoma- A Triple Whammy Strategical Surgical Approach Using Da Vinci Robot
Fibroids, endometriosis and adenomyosis have similar etiopathogenesis. All are estrogen-dependent and may present simultaneously in some women. The coexistence of endometriosis, adenomyosis and leiomyoma is poorly understood (1)(2). The current literature reports an incidence of concomitant fibroids and endometriosis between 12 and 20% (3)(4). Nezhat reported that 87.1% of patients with symptomatic fibroids also had histology-proven endometriosis, thus there is a need for concomitant diagnosis and intraoperative treatment (5).
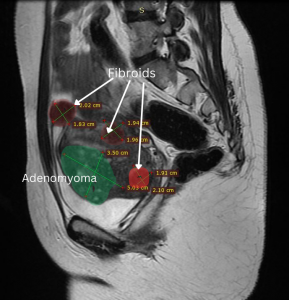
We report on a 29-year-old, nulligravida, patient who presented with severe dysmenorrhea (VAS 10) and heavy menstrual bleeding, dyspareunia and primary infertility. Her MRI showed an adenomyoma (5×3.5 cm), multiple fibroids (FIGO-3-5), left hematosalpinx, and bilateral endometriomas. Her CA125 was 655.04 ng/dl and AMH 1.32. Robot assisted intervention was planned for pain relief and fertility enhancement. The technical challenge was to remove all the fibroids and adenomyoma in the setting of stage 4 endometriosis. A systematic approach was planned to this complex pathology in six steps.
Step one was preoperative planning using MRI images (Figures 1 & 2).
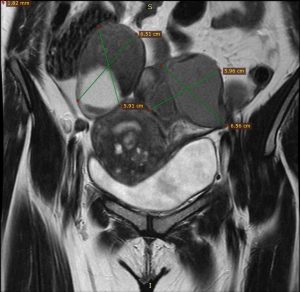
Figure 1 Figure 2
The second step was anatomical restoration of the posterior compartment. This began with identification of both ureters at pelvic brim, bilateral ovariolysis and rectal adhesiolysis with excision of lesion from rectovaginal septum. Diluted vasopressin (20 units in 200ml saline) was then infiltrated into the uterus as well as the endometrioma capsule.
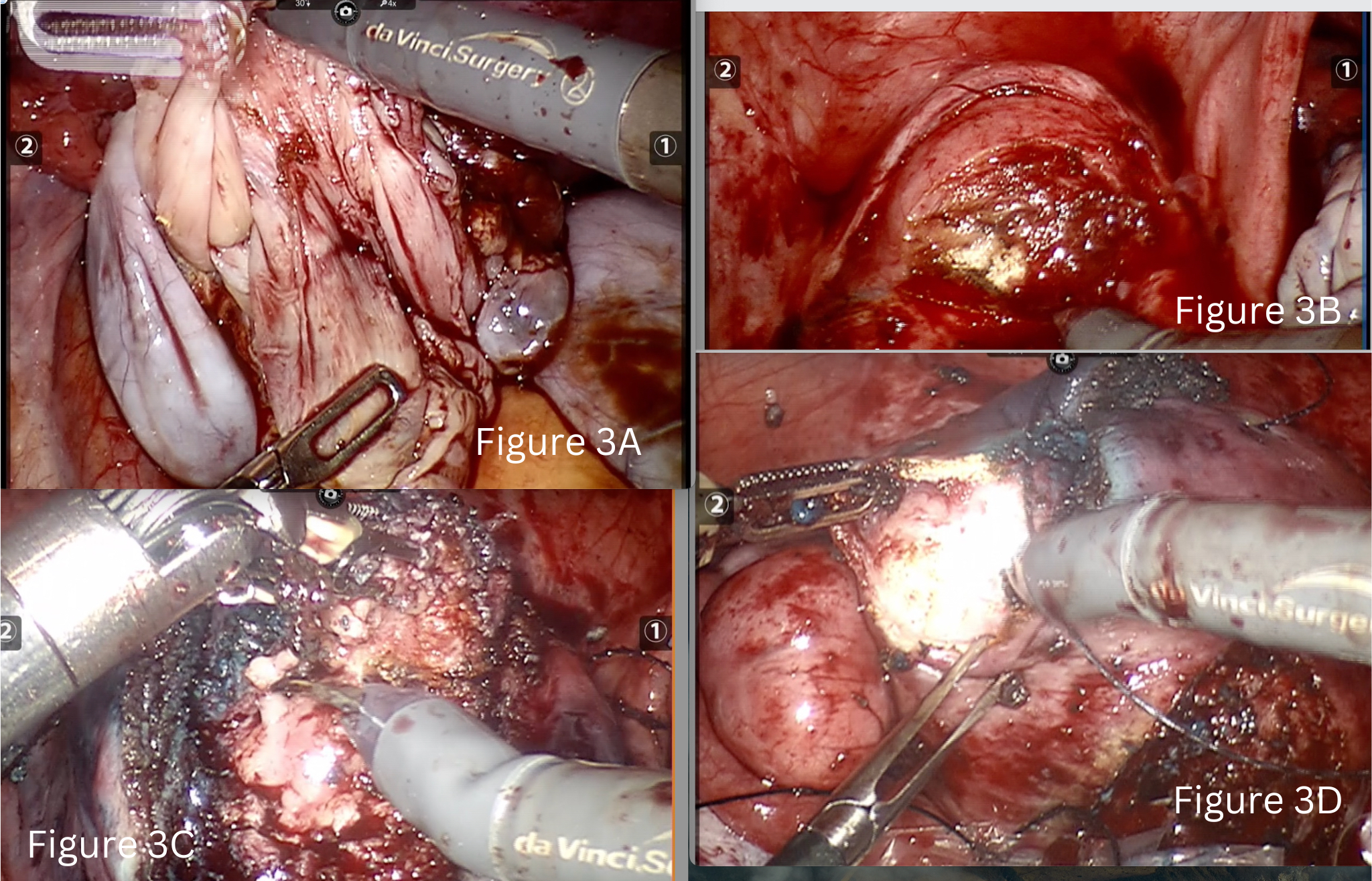
We performed bilateral ovarian cystectomy along with tubal patency test (Fig 3A). The right tube was patent, and the left hematoslapinx was excised. An endo-bag was inserted at this point to collect all the specimen as the surgery progressed.
Step four was a sequential approach from anterior uterine wall to posterior. We began with removal of lower anterior wall myoma (3×3 cm FIGO 5) and uterine reconstruction immediately (Fig 3B).
In the fifth step we did adenomyomectomy (5×3.5 cm) involving the fundo-anterior area of the uterus. This was performed by identifying the borders of adenomyotic foci and by giving a longitudinal incision on it. Sharp and delicate dissection of the adenomyoma from the surrounding relatively healthy myometrium tissue was done using monopolar hot shears and effort was made to preserve of maximal residual serosa or serosa-muscular layer. Methylene blue dye was injected through the Rumi manipulator at this stage and a breach of 1 cm identified (Fig 3C).
Closure and reconstruction of uterine wall and serosa with 1-0 barbed suture was done using a mega needle driver. We did not suture the endometrium separately.
Sixth step was to then to move towards the posterior wall and remove the remaining three fibroids followed by uterine reconstruction of the posterior wall of the uterus (Fig 3D).
The endometriosis and adenomyosis were more severe in this patient when compared to fibroids which were smaller in size. A similar finding was reported by Nezhat and group where women with smaller fibroids had more severe endometriosis (5). It is possible that women with isolated fibroids may be able to avoid surgical intervention until they grow in size and symptoms become unbearable. But, women with severe endometriosis are more symptomatic and undergo surgery sooner as compared to patients with isolated fibroids.
Images Legend:
Figure 1- Bilateral Endometrioma in MRI coronal section
Figure 2- Adenomyoma and posterior wall fibroids in sagittal section
Figure 3 A-Cystectomy of left ovarian endometrioma.
Figure 3 B –Myomectomy of fibroid located in lower anterior wall of uterus.
Figure 3 C- Adenomyomectomy
Figure 3 D- Myomectomy of fibroid located in posterior wall of uterus.
References:
- Toriyama A, Ishida M, Amano T, Nakagawa T, Kaku S, Iwai M, et al. Leiomyomatosis peritonealis disseminata coexisting with endometriosis within the same lesions: a case report with review of the literature. Int J Clin Exp Pathol. 2013 Nov 15;6(12):2949–54.
- Tanmahasamut P, Noothong S, Sanga-Areekul N, Silprasit K, Dangrat C. Prevalence of endometriosis in women undergoing surgery for benign gynecologic diseases. J Med Assoc Thail Chotmaihet Thangphaet. 2014 Feb;97(2):147–52.
- Uimari O, Järvelä I, Ryynänen M. Do symptomatic endometriosis and uterine fibroids appear together? J Hum Reprod Sci. 2011;4(1):34–8.
- Fuldeore M, Yang H, Du EX, Soliman AM, Wu EQ, Winkel C. Healthcare utilization and costs in women diagnosed with endometriosis before and after diagnosis: a longitudinal analysis of claims databases. Fertil Steril. 2015 Jan;103(1):163–71.
- Nezhat C, Li A, Abed S, Balassiano E, Soliemannjad R, Nezhat A, et al. Strong Association Between Endometriosis and Symptomatic Leiomyomas. JSLS. 2016;20(3): e2016.00053.
About the Authors:

Rooma Sinha, MD, DNB, MNAMS, FICOG
Bana Rupa, MBBS, MS
Dr. Sinha is a Member of the AAGL Fibroids SIG. Dr. Sinha and Dr. Rupa are Minimal Access and Robotic Surgeons of Gynecology at Apollo Health City in Hyderabad, India.
Technology Update: Precision Medicine for Uterine Fibroid (UF) Management – The Dawn of a New Era
The variability of UF symptomatology is a unique and poorly understood feature of the disease. Furthermore, the unpredictability of UF response to medical therapy limits the clinician’s ability to tailor and personalize management plans (1). Resultantly, UFs are treated as a single disease, with management decisions dependent on the availability of treatment options, patient symptoms, and patient/physician preferences (2).
Precision medicine is a new approach to medicine that offers tailored treatments to affected populations based on an increased understanding of the molecular mechanism of the disease in question (3). If this follows, then an increased understanding of the molecular mechanism of UFs may give us some insight into their specific clinical manifestations and perhaps their ability to respond to different therapies. This knowledge could then guide our ability to design patient and fibroid-specific therapies for UF management and improve clinical outcomes and patient experience.
UFs are associated with a high extracellular matrix (ECM) accumulation, which explains the stiff characteristics of these tumors (4). ECM not only plays a role in the structure, stiffness, and cell support of UFs but also serves as a reservoir of growth factors and bioactive molecules (5). TGF-β is an important cytokine that is stored in the ECM. It is stored in a biologically inactive form and when activated it increases the accumulation of ECM in the UF (6). Studies have demonstrated that UF stiffness directly affects the growth of the tumor through the induction of fibrosis (4,7,8). Fibrosis in turn, affects cell growth rate by a phenomenon called mechanotransduction in which mechanical forces i.e., stiffness applied to cells are translated into chemical signaling that elicit adaptive responses (4,9). Since UF tissue stiffness intimately interplays with UF tumor cell signal transduction and tumor growth, it is a reasonable deduction that UF stiffness may closely reflect tumor biological behavior. Therefore, UF stiffness may provide some insight into the severity of UF clinical manifestations and response to medical therapy.
A relatively new technology in the field of sonography, shear wave elastography (SWE) is an objective quantitative method that assesses the stiffness of an anatomic structure to provide tissue characterization. SWE has been validated against traditional material testing techniques (10,11) and can easily be incorporated into a standard ultrasound exam. This relatively new technology is currently being used as standard practice for diagnosis and prognosis of liver cirrhosis (12), breast masses (13), and incompetent cervix (14), with a major positive impact on patients’ outcome since its introduction in the past decade.
Despite these advantages, the clinical applicability of using SWE in gynecology has not yet been realized. Only a few reports of SWE use in gynecology and specifically for UF have been published with encouraging results (15,16,17). With our rapidly growing understanding about the link between UF morphology and tumor behavior, the successful incorporation of SWE into gynecological practice may be just what we need to usher us into the new era of precision medicine for UF management.
References:
- Brito, L.G. et al. (2014) ‘Uterine Leiomyoma: Understanding the impact of symptoms on womens’ lives’, Reproductive Health, 11(1). doi:10.1186/1742-4755-11-10.
- Vilos, G.A. et al. (2015) ‘The management of uterine leiomyomas’, Journal of Obstetrics and Gynaecology Canada, 37(2), pp. 157–178. doi:10.1016/s1701-2163(15)30338-8.
- Precision medicine (no date) EFPIA Homepage. Available at: https://www.efpia.eu/about-medicines/development-of-medicines/precision-medicine/ (Accessed: 08 July 2023).
- Leppert, P.C., Jayes, F.L. and Segars, J.H. (2014) ‘The extracellular matrix contributes to mechanotransduction in uterine fibroids’, Obstetrics and Gynecology International, 2014, pp. 1–12. doi:10.1155/2014/783289.
- Ali, M. et al. (2023) ‘Prevention of uterine fibroids: Molecular mechanisms and potential clinical application’, Journal of Endometriosis and Uterine Disorders, 1, p. 100018. doi:10.1016/j.jeud.2023.100018.
- Ciebiera, Michał et al. (2017) ‘Role of transforming growth factor β in uterine fibroid biology’, International Journal of Molecular Sciences, 18(11), p. 2435. doi:10.3390/ijms18112435.
- Rafique, S., Segars, J. and Leppert, P. (2017) ‘Mechanical signaling and extracellular matrix in uterine fibroids’, Seminars in Reproductive Medicine, 35(06), pp. 487–493. doi:10.1055/s-0037-1607268.
- Cordeiro Mitchell, C.N. et al. (2021) ‘Mechanical stiffness augments ligand-dependent progesterone receptor B activation via MEK 1/2 and Rho/rock–dependent signaling pathways in uterine fibroid cells’, Fertility and Sterility, 116(1), pp. 255–265. doi:10.1016/j.fertnstert.2020.12.011.
- Yang, Q., Ciebiera, M., Bariani, M.V., Ali, M., Elkafas, H., Boyer, T.G. and Al-Hendy, A., (2022) Comprehensive review of uterine fibroids: developmental origin, pathogenesis, and treatment. Endocrine reviews, 43(4), pp.678-719.
- Eby, S.F. et al. (2013) ‘Validation of shear wave elastography in skeletal muscle’, Journal of Biomechanics, 46(14), pp. 2381–2387. doi:10.1016/j.jbiomech.2013.07.033.
- Chatzistergos, P.E. et al. (2018) ‘Shear wave elastography can assess the in-vivo nonlinear mechanical behavior of heel-pad’, Journal of Biomechanics, 80, pp. 144–150. doi:10.1016/j.jbiomech.2018.09.003.
- Thiele, M. et al. (2016) ‘Transient and 2-dimensional shear-wave elastography provide comparable assessment of alcoholic liver fibrosis and cirrhosis’, Gastroenterology, 150(1), pp. 123–133. doi:10.1053/j.gastro.2015.09.040.
- Cosgrove, D.O. et al. (2011) ‘Shear wave elastography for breast masses is highly reproducible’, European Radiology, 22(5), pp.1023–1032. doi:10.1007/s00330-011-2340-y.
- Gennisson, J.-L. et al. (2011) ‘Shear wave elastography in obstetrics: Quantification of cervix elasticity and uterine contraction’, 2011 IEEE International Ultrasonics Symposium [Preprint]. doi:10.1109/ultsym.2011.0519.
- Furukawa, S. et al. (2014) ‘The measurement of stiffness of uterine smooth muscle tumor by elastography’, SpringerPlus, 3(1). doi:10.1186/2193-1801-3-294.
- Frank, M. et al. (2015) ‘Importance of transvaginal elastography in the diagnosis of uterine fibroids and adenomyosis’, Ultraschall in der Medizin – European Journal of Ultrasound, 37(04), pp. 373–378. doi:10.1055/s-0035-1553266.
- Wang, X.L., Lin, S., and Lyu, G.R., (2022) Advances in the clinical application of ultrasound elastography in uterine imaging. Insights into Imaging, 13(1), p.141.
About the Author:
Sandra Madueke-Laveaux, MD, MPH

The post Spotlight On: Fibroids appeared first on NewsScope.